INTRODUCTION
More than 2 years after WHO declared COVID-19 a pandemic,1 the world is facing its impacts on different life consequences.
In addition to Morbidity and Mortality, Long term effects of the disease were founded and described as a post-COVID-19 syndrome, within which patients are still complying with signs and symptoms for several weeks from acute infection.2
About 60 days after the onset of the first COVID-19 symptom, it is evident that only 13% of COVID-19 patients were free of the symptoms of COVID-19, and 32% had one to two symptoms, while 55% experienced more than three symptoms.3
Clinicians are observing and reading reports of patients with persistent severe symptoms, but because COVID-19 is a new disease, so most of the clinical course remains uncertain, as well as the possible long-term health concerns. CDC described the post-COVID syndrome as symptoms that develop and last for four weeks or even months and cannot be explained by an alternative diagnosis.4
According to COVID- the 19 symptoms study, in the United Kingdom, Sweden, and the United States, more than four million people have shown symptoms after being diagnosed with COVID-19 and defined post-COVID-19 as the existence of symptoms after 21 days from the first symptoms, post-COVID-19 symptoms may remain for >3 months Greenhalgh.5
The cause of persistent symptoms is unclear, but several different disease mechanisms are likely to be involved, including an inflammatory response with a vasculitis component.6
The most commonly reported symptoms after acute COVID-19 are fatigue, dyspnea, and joint and chest pain; in addition to these general symptoms, multiple organ failure has been recorded, including the heart, lungs, and brain. From a pathogenesis standpoint, these complications could be the consequence of direct tissue invasion by the virus, inflammation, cytokine storm, and coagulation abnormality in association with COVID-19, or a combination of these factors.3
Even with the assertion of the WHO’s primary health care (PHC) role to recognize many health issues, there is a shortage of studies in developing countries to estimate the prevalence of COVID-19 common presenting symptoms and post-COVID-19 symptoms at PHC compared to those found in western countries. Therefore, there is a need for a scientific search to be aware of these symptoms to fill up a gap in the evidence base and make a basis for additional discussion.
This study aimed to:
-
Estimate the prevalence of residual symptoms among a sample of people infected with COVID-19 who survive and recover.
-
To find out the relation between the severity of the disease and post-COVID-19 syndrome.
-
To know the incidence of post-COVID-19 syndrome in chronic disease patients.
-
Collect and review data on this emergent disease to build a piece of knowledge and enhance future care.
MATERIALS AND METHODS
This cross-sectional study was conducted among patients who recovered from COVID-19, aged ≥ 18 years, who attended 6 primary health care centers in 6 municipalities in Erbil City-Iraq, and who complained of symptoms during the acute infection period. Twenty-four PHCs provide six hours a day of health services for 6 municipalities in the city of Erbil, 6 health care centers are randomly selected, one from each municipality. The primary health care centers (PHCs) are Shady, Brayatti, Mala Afandi, Nazdar Bamarni, Shahidan, and Ankawa PHCs, based on the geographic distribution in Erbil/Kurdistan Region/ Iraq. The study duration was from the 1st of March 2021 till the end of March 2022.
A convenience sample of randomly selected 300 patients was involved in the study, and all the patients capable of participating were interviewed after obtaining their verbal and written informed consent.
Inclusion criteria included all Patients infected with COVID-19, diagnosed at least four weeks before, and resolved according to CDC definition. Data were obtained by a standardized structured interview questionnaire adapted by the supervisor and researcher. The questionnaire was composed of three parts, the first part was related to sociodemographic data of participants like age, education, occupation, blood group, weight, and height for body mass index calculation, second part collected information about the general health status like regular physical activity, smoking status, and any chronic disease. In contrast, the last part was a disease-related question like diagnosis method, time since diagnosis, type of health care received, disease severity, and persistent symptoms after the recovery.
SPSS conducted statistical analysis for social science programs and was used for entering the data, and results were analyzed through frequency and percentage.
The results were checked for normality using frequency distribution, t-test, and Chi-square. P–values ≤ 0.05 will be considered statistically significant.
RESULTS
We enrolled 300 participants in the current study, Table 1 and Figure 1 show that most (62%) of the participants were married, more than half (54%) of the study groups were female, 63% of them were urban residential, nearly half (50.4%) of respondents had medium Socio-economic status, most (57%) of the were non-smokers while 31% were smokers, the maximum amount 35% of them had AB blood type, followed by 34% of B blood group; finally 48.6% of participants were active physically, and 39.4% of them had a sedentary lifestyle.
TABLE 1. General characteristics of the participants.
| Variables | Categories | Frequency | Percent |
|---|---|---|---|
| Marital status | Married | 186 | 62 |
| Single | 86 | 28.7 | |
| Divorced | 28 | 9.3 | |
| Gender | Male | 138 | 46 |
| Female | 162 | 54 | |
| Residence | Rural | 111 | 37 |
| Urban | 189 | 63 | |
| Socio-economic status | Low | 61 | 20.3 |
| Medium | 151 | 50.4 | |
| High | 88 | 29.3 | |
| Smoking | Smoker | 93 | 31 |
| Non-smoker | 171 | 57 | |
| Ex-smoker | 36 | 12 | |
| Blood group | A | 75 | 25 |
| B | 102 | 34 | |
| AB | 105 | 35 | |
| O | 18 | 6 | |
| Physical activity | Athletic | 35 | 11.7 |
| Active | 146 | 48.6 | |
| Sedentary life | 119 | 39.7 | |
| Total | 300 | 100 | |
FIGURE 1. Socio-economic status.
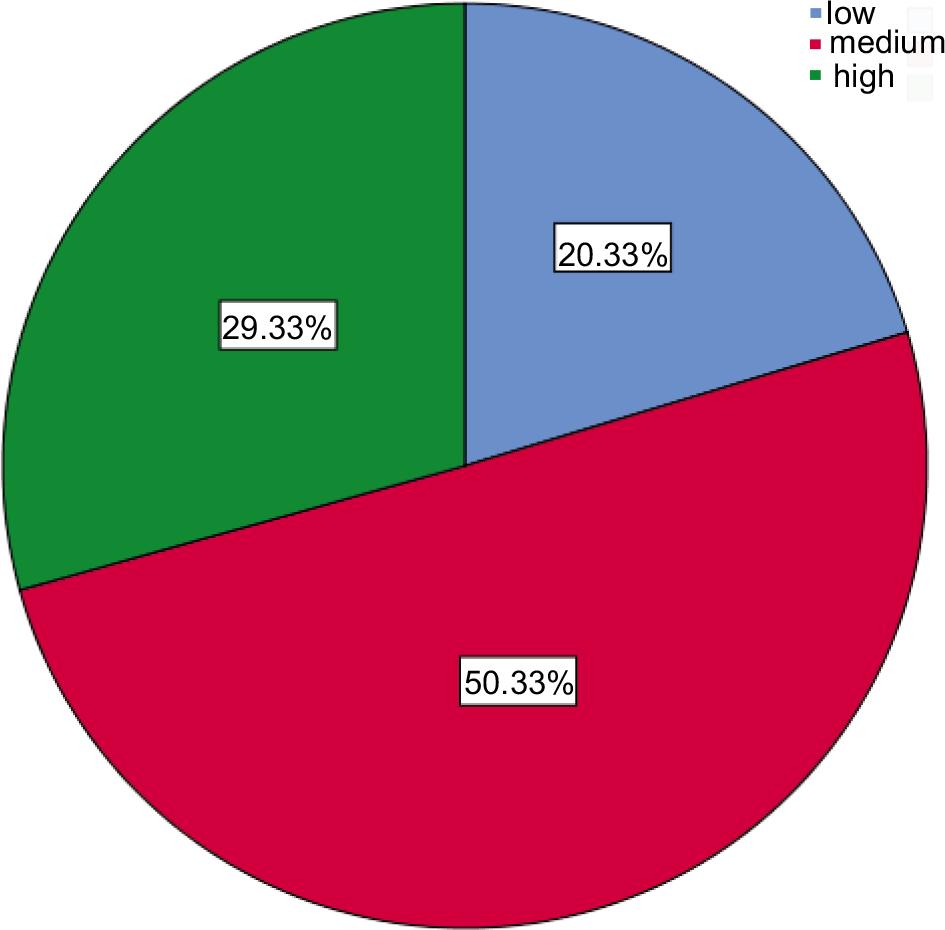
Table 2 determines that the mean age ± S.D of participants was 50.3 ± 16.13 years, the average weight ± S.D of respondents was 78.1 ± 14.46 Kg, and the mean height ± S.D of study groups was 163.3 ± 9.05 cm.
TABLE 2. The average age, weight, and height.
| Variables | N | Range | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|---|
| Age in years | 300 | 69 | 18 | 87 | 50.3 | 16.13 |
| Weight (Kg) | 300 | 75 | 45 | 120 | 78.1 | 14.46 |
| Height (cm) | 300 | 44 | 140 | 184 | 163.3 | 9.05 |
Findings of Table 3 show that more than half (53%) of respondents had a chronic disease, 12% of patients suffered from CHD, only 2% of them faced kidney failure, 39% of subjects experienced hypertension, 13% of them had diabetes mellitus, only 4% of patients got through stroke, 6% of them had cancer and finally 13% of patients infected by COPD.
TABLE 3. Chronic disease of participants.
| Variables | Categories | Frequency | % |
|---|---|---|---|
| Chronic disease | yes | 159 | (53.0) |
| CHD | yes | 36 | (12.0) |
| Kidney failure | yes | 6 | (2.0) |
| Hypertension | yes | 117 | (39.0) |
| Diabetes mellitus | yes | 39 | (13.0) |
| Stroke | yes | 12 | (4.0) |
| Cancer | yes | 18 | (6.0) |
| COPD | yes | 39 | (13.0) |
Results of Table 4 reveal that 46% of samples diagnosed by rapid test, most (62%) of patients infected by mild symptoms of COVID-19, nearly one-third (32%) had a high level of ferritin, 41% of study samples experienced the disease more than 14 days, more than half (53%) of SPO2 readings were (96-100%), 46% of patients were taken care by nurses.
TABLE 4. General COVID-19 characteristics of participants.
| Variables | Categories | Frequency | Percent |
|---|---|---|---|
| COVID-19 diagnosed by | Rapid test | 138 | (46.0) |
| PCR | 84 | (28.0) | |
| Clinical by a specialist | 78 | (26.0) | |
| COVID-19 severity | Mild symptoms | 186 | (62.0) |
| Pneumonia diagnosed | 75 | (25.0) | |
| Hospital admission | 39 | (13.0) | |
| Ferritin level | Normal | 39 | (13.0) |
| High | 96 | (32.0) | |
| Unknown | 165 | (55.0) | |
| Disease duration | <7 days | 114 | (38.0) |
| 7–14 days | 63 | (21.0) | |
| >14 days | 123 | (41.0) | |
| SPO2 level | 96–100% | 159 | (53.0) |
| 90–95% | 78 | (26.0) | |
| >90% | 63 | (21.0) | |
| Health care type | General practitioner | 15 | (5.0) |
| Medical specialist | 114 | (38.0) | |
| Nurse | 138 | (46.0) | |
| Pharmacist nothing | 33 | (11.0) | |
| Total | 300 | 100 | |
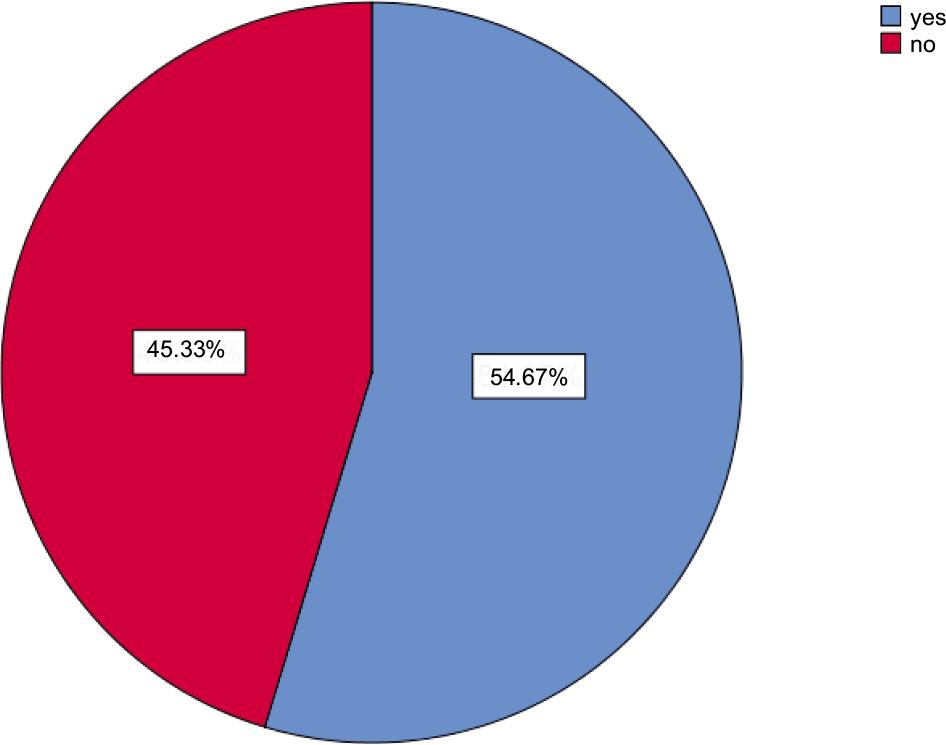
Outcomes in Table 5 and Figure 2 show the symptoms and signs of COVID-19 as follows, 6% of participants faced chest tightness, 11% of patients struggled with dyspnea, 12% of them experienced joint pain, (3%) for each case caught chest pain, anosmia, and vertigo, 14% for each of respondents got cough and sputum, only 2% of them faced redness of eyes, 30% of patients had a headache, 13% of cases experienced a loss of appetite, 5% of them got through a sore throat, 7% of participants got myalgia, more than one third (35%) felt fatigued, 19% of them had poor concentration, more than half (45.3%) of patients did not experience any persistent symptoms, in reverse the rest of the patients had persistent symptoms; 11.7% of them had 1, 26% had 2–3, and finally 17% had >3 persistent symptoms.
TABLE 5. Symptoms and signs of COVID-19 infection.
| Variables | No. | (%) N=300 |
|---|---|---|
| Chest tightness | 18 | (6.0) |
| Dyspnea | 33 | (11.0) |
| Joint pain | 36 | (12.0) |
| Chest pain | 9 | (3.0) |
| Cough | 42 | (14.0) |
| Anosmia | 9 | (3.0) |
| Red eyes | 6 | (2.0) |
| Headache | 90 | (30.0) |
| Sputum production | 42 | (14.0) |
| Lack of appetite | 39 | (13.0) |
| Sore throat | 15 | (5.0) |
| Vertigo | 9 | (3.0) |
| Myalgia | 21 | (7.0) |
| Fatigue | 105 | (35.0) |
| Poor concentration | 57 | (19.0) |
| No persistent symptoms | 136 | (45.3) |
| 1 persistent symptom | 35 | (11.7) |
| 2–3 persistent symptoms | 78 | (26.0) |
| ˃3 persistent symptoms | 51 | (17.0) |
FIGURE 2. Persistent symptoms.
Findings of Table 6 determine that there was a significant statistical association between persistent symptoms and severity of COVID-19; 46.2% of cases with post-COVID-19 syndromes had mild symptoms, while the majority (92.3%) of patients with Persistent symptoms were admitted to hospital, and p-value was 0.001. There was significant statistical association between persistent symptoms and chronic disease, most (67.9%) respondents with post-COVID-19 syndromes suffered from chronic illness. A lower amount (39.7%) of patients with COVID-19 symptoms did not have a chronic disease, and the p-value was 0.001. There was a significant statistical association between persistent symptoms and CHD; most (83.3%) post-COVID-19 syndrome cases experienced CHD. On the contrary, nearly half (50.8%) of them did not get CHD, and p-value was 0.001.
TABLE 6. Association between the severity of the disease and post-COVID-19 syndrome.
| Variables | Categories | Persistent symptoms | p-value | |
|---|---|---|---|---|
| Yes No. (%) | No No. (%) | |||
| Severity of COVID-19 | Mild symptoms | 86 (46.2) | 100 (53.8) | 0.001 |
| Pneumonia | 42 (56.0) | 33 (44.0) | ||
| Hospital admission | 36 (92.3) | 3 (7.7) | ||
| Chronic disease | yes | 108 (67.9) | 51 (32.1) | 0.001 |
| CHD | yes | 30 (83.3) | 6 (16.7) | 0.001 |
| Kidney failure | yes | 1 (16.7) | 5 (83.3) | 0.095 |
| Hypertension | yes | 71 (60.7) | 46 (39.3) | 0.094 |
| Diabetes mellitus | yes | 37 (94.9) | 2 (5.1) | 0.001 |
| stroke | yes | 12 (100) | 0 (0.0) | 0.001 |
| Cancer | yes | 15 (83.3) | 3 (16.7) | 0.012 |
| COPD | yes | 22 (56.4) | 17 (43.6) | 0.815 |
| Gender | male | 84 (60.9) | 54 (39.1) | 0.049 |
| female | 80 (49.4) | 82 (50.6) | ||
| Socio-economic status | low | 33 (54.1) | 28 (45.9) | 0.681 |
| medium | 86 (57.0) | 65 (43.0) | ||
| high | 45 (51.1) | 43 (48.9) | ||
There was a statistically significant association between persistent symptoms, and the vast majority (94.9%) of participants with persistent syndromes had DM. In comparison, less than half (48.7%) of them did not have DM, and the p-value was 0.001. There was a statistically significant association between persistent symptoms and stroke; all (100%) cases with post-COVID syndromes got a stroke. In comparison, nearly half (52.8%) of them faced stroke, and the p-value was 0.001. There was a statistically significant association between persistent symptoms and CA, the majority (83.3%) of post-COVID syndrome respondents had CA. In comparison, 52.8% did not get CA, and the p-value was 0.012. There was a statistically significant association between persistent symptoms and gender; most (60.9%) cases with Persistent symptoms were male, while 49.4% were female. A Chi-square test was done, and the p-value was 0.049. There was a non-significant statistical association between persistent symptoms and kidney failure, HTN, COPD, and socioeconomic status. Chi-square was done an p-values were 0.095, 0.094, 0.815 and 0.681 respectively.
Conclusions of Table 7 reveal a significant statistical association between persistent symptoms and disease duration; most (73%) of cases with post-COVID-19 syndrome got the disease from 7-14 days, while 54.5% of them struggled with COVID-19 >14 days. There was a significant statistical association between persistent symptoms and PO2 levels, the majority (84.1%) of samples with persistent symptoms had low oxygen levels (>90%) while 48.4% of them had high oxygen levels (96–100%). A Chi-square test was done, and the p-value for both was 0.001. There was a non-significant statistical association between persistent symptoms and smoking, blood groups, Physical activity, and ferritin level. Chi-square test was done and p-values were 0.694, 0.118, 0.211, and 0.117 respectively.
TABLE 7. Association between some other variables and post-COVID-19 syndrome.
| Variables | Categories | Persistent symptoms | p-value | |
|---|---|---|---|---|
| yes | no | |||
| Smoking | Smoker | 54 (58.1) | 39 (41.9) | 0.694 |
| Non-smoker | 90 (52.6) | 81 (47.4) | ||
| Ex-smoker | 20 (55.6) | 16 (44.4) | ||
| Blood groups | A | 45 (60.0) | 30 (40.0) | 0.118 |
| B | 51 (50.0) | 51 (50.0) | ||
| AB | 62 (59.0) | 43 (41.0) | ||
| O | 6 (33.3) | 12 (66.7) | ||
| Physical activity | Athletic | 16 (45.7) | 19 (54.3) | 0.211 |
| Active | 87 (59.6) | 59 (40.4) | ||
| Sedentary life | 61 (51.3) | 58 (48.7) | ||
| Ferritin level | Normal | 27 (69.2) | 12 (30.8) | 0.117 |
| High | 53 (55.2) | 43 (44.8) | ||
| Unknown | 84 (50.9) | 81 (49.1) | ||
| Disease duration | less than 7 days | 51 (44.7) | 63 (55.3) | 0.001 |
| 7-14 days | 46 (73.0) | 17 (27.0) | ||
| more than 14 days | 67 (54.5) | 56 (45.5) | ||
| SPO2 level | 96-100% | 77 (48.4) | 82 (51.6) | 0.001 |
| 90-95% | 34 (43.6) | 44 (56.4) | ||
| ˂ 90% | 53 (84.1) | 10 (15.9) | ||
DISCUSSION
In the last 20 years, several respiratory-related viral diseases such as SARS-CoV, H1N1 influenza, and MERS-CoV epidemics have been outbreaks. Although previous coronaviruses, SARS-CoV and MERS-CoV, exhibited a high death rate of 9.6% and 35%, respectively, SARS-CoV-2 was declared a pandemic because of its high contagiousness and global spreading.7 COVID-19 has a wide range of disorders, from mild disease to death. Difficulty breathing and fever are the most common signs. Furthermore, malaise and shortness of breath have been reported as symptoms. Symptoms might appear from 2 days to 2 weeks after infection.8
The COVID-19 infection may have long-term effects known as a post-COVID syndrome, including post-sepsis and post-ICU syndrome. Wide variations in estimates of the incidence and prevalence of post-COVID-19 syndrome have been recorded since it is neither a homogeneous nor singular entity. The most often reported complaint, chronic weariness, frequently manifests without objective respiratory function abnormalities or fibrosing lung lesions.
Chronic fatigue, dyspnea, shortness of breath, chest pains, headaches, loss of smell or taste, and muscle and joint pain were the most commonly reported long-term symptoms in COVID-19 patients, followed by depression, anxiety, insomnia, itchy body, heart palpitations, tachycardia, anorexia, tingling fingertips, and brain fog.9–11 This study described the persisting symptom and post-COVID-19 syndrome for clinical features among 300 confirmed COVID-19 patients. Generally, 46% were male, and 54% were female. The mean age was 50.3 years in the range of 69 years, and in the range of 75 kg, the average weight was 78.1 ± 14.46 Kg.
In this study, >50% of the patients suffered from chronic diseases, 88% did not have CHD, and just 2% suffered from kidney disease. They don’t suffer from hypertension and diabetes mellitus. Most patients did not have (stroke, cancer, and COPD).
In Table general COVID-19 characteristics of participants were studied. Rapid tests for diagnosis of COVID-19 were used by <50% of patients, while some depended on PCR and clinical by a specialist. More than half of the patients have mild symptoms. The Ferritin level in 55% was unknown, while nearly one-fourth of the patients suffering from the disease >14 days. More than half their SPO2 level was 96–100%. Less than half of patients have nurse as health care type.
Generally, most patients presented (chest tightness, dyspnea, joint pain, chest pain, cough, anosmia, red eyes, headache, sputum production, lack of appetite, sore throat, vertigo, myalgia, fatigue, and poor concentration).12 In this study, one-third of the patients were suffering from fatigue commonly reported with follow-up studies in the United Kingdom, Italy and Bangladesh, which also revealed that fatigue is the most frequent symptom among individuals with post-Covid-19.13,14 Figure 2 shows that in a total of 300 participants, 54.67% suffered from persistent symptoms, which means the result was significant for persistent symptoms. If we compare our data to data in the review, we can see the presence of patients with persistent symptoms is higher.15 It is an excellent point to insure our data.
Next, the p-value was calculated for the association between disease severity and post-COVID-19 syndrome. The result was significant for pneumonia, chronic disease, and CHD. The persistent symptoms were >50% for pneumonia while 92.3% of patients required hospital admission.16,17 More than half of patients with chronic disease developed post-COVID-19 syndrome. Most of the participating CHD patients were at high risk for post-COVID-19 syndrome. This was emphasized in the literature also, which supports our data.18 Mostly all patients with diabetes mellitus there was under the dangerous to post COVID-19 syndrome.19 Other patients suffering from disease like stroke and cancer also facing risk with COVID-19 and they strongly developed it. Disease like kidney failure, hypertensions and COPD there results was non-significant.
We conducted the p-value calculation in our study also to see the effect of gender and socioeconomic status. The first one gives significant effect and the second one non-significant. 60.9% of male were developing post-COVID-19 syndrome. One plausible reason for the gender differences could be due to hormonal differences. Female has shown higher incidence rate than male.8
Lastly, In Table 7 the data shows the results were non-significant for smoker patients when we compare the persistent symptom and duration of COVID-19 disease. This data was in reverse to what was found in review.20 We also found that factors like (blood groups, physical activity, ferritin level was non-significant to its p-value calculation. To date, it is still a matter of debate if disturbances of iron handling are just a reflection of the physiological adaption to the infectious disease or if dysregulated iron homeostasis contributes to COVID-19 pathobiology and disease outcome.21 The latter assumption is supported by the observation that hyperferritinemia is associated with increased mortality in COVID-19. Mechanistically, it has been suggested that hyperferritinemia and hepcidin dysregulation are related to iron toxicity and may contribute to end-organ damage in COVID-19.22 This is a limitation of our work because according to data obtained and calculation of p-value, >50% of the patients were suffering from persistent symptoms >4 weeks. All these data depended on the p-value and Chi-square test.23–25
CONCLUSIONS
Most people who have recovered from COVID-19 have a wide range of long-lasting symptoms that make it hard to go about their daily lives. This is now called post-COVID-19 syndrome, which could have been caused by several things. Age, gender, whether they already have health problems are all things to consider. Even though all COVID-19 victims should be watched for a long-term evaluation and treatment of post-COVID symptoms.
After 4 weeks, symptoms persisted in >50% of the individuals in this study cohort who had mild COVID-19 and did not require hospitalization. This was the finding of a study that examined patients in groups. SPO2, ferritin level, and lower health status were all significantly correlated with persistent symptoms. This is true even though the rates of long-term morbidity reported here were lower than those in cohorts of patients who were hospitalized. A complex illness called post-acute-COVID-19 comprises various enduring symptoms. These symptoms are frequently explained by persistent cardiac problems, these symptoms can also be caused by mental health issues, the degree of which seems to be correlated with the initial severity of COVID-19 and the amount of time that has gone by since the acute infection. The functional status and quality of life of the countless individuals affected by post-acute-COVID-19 are significantly impacted.