Introduction
Human immunodeficiency virus (HIV) attacks the body’s immune system, specifically the CD4 cells. In 2017, 36.9 million people globally were living with HIV, and 59% of all people living with HIV (PLHIV) were provided with antiretroviral therapy (ART) 1. Thailand, one of the countries with the highest prevalence of HIV in Asia and the Pacific, accounts for 9% of the region’s total population of PLHIV 2. HIV incidence per 1000 population was 0.09 [0.08 – 0.10]. PLHIV were 480,000, and 75% of them are on ART 3. The incidence rate of metabolic syndrome (MetS) in patients on ART was 1.2 per 100 person-months, and most of them had a low level of high-density lipoprotein (HDL) 4.
The development of highly active antiretroviral therapy (HAART), a treatment regimen of combined ART, improved the quality of life and increased the life expectancy of PLHIV 5. Only 49.6% of the deaths were due to acquired immunodeficiency syndrome while the rest was due to age-related illness, especially cardiovascular disease 6. This increases the importance of age-related illness among these patients 6. In the era of HAART, several traditional risk factors of MetS are presented, which finally lead to MetS, cardiovascular events, and mortality 5, 7. In addition, both HIV infection and ART can cause metabolic abnormalities 8. The previous study showed that the highest prevalence of metabolic component in patients treated with the first-line HAART group and the ART-naive group were 53.2 and 14.8%, respectively 9. Prevalence of MetS in PLHIV, ranging from 11.2 up to 45.4%, was reported 7, and 24.2% of patients on first-line HAART regimens were diagnosed with MetS 9. Furthermore, MetS prevalences across subgroups were as follows; 23.7 and 26.7% in men and women, respectively, and 25.8, 17.2, and 17.9% in protease inhibitor (PI) users, nonnucleoside reverse transcriptase inhibitor (NNRTI) users, and nucleoside reverse transcriptase inhibitors (NRTI) users, respectively. However, there is no significant difference in the prevalence of MetS in high or low proportion of smokers, 8% versus 22.2%, P = 0.193 10.
Dyslipidemia, insulin resistance, and diabetes mellitus (DM) have been observed among patients treated with HAART, especially PIs 5. Furthermore, the annual cumulative incidence of events related to coronary artery disease was significantly higher in patients treated with PI than patients treated with other ART 5. Therefore, the World Health Organization (WHO) recommended first-line ART regimens for adults consisting of two NRTIs plus one NNRTI 11. However, it was reported that NRTI and NNRTI can increase the risk of DM 12. Dyslipidemia has been observed in NNRTI-based therapy, although to a lesser degree when compared to PI 13. Therefore, prevention of MetS by monitoring metabolic components appears to be an effective strategy. However, there are some limitations in this regard in low- and middle-income countries.
According to the universal health coverage in Thailand, the benefit package for patients with HIV is the free basic medical services available to all HIV patients. The basic blood chemistry testing, consisting of fasting plasma glucose (FPG) , serum total cholesteral, and serum triglyceride, have been performed in patients with HIV taking into consideration age and comorbidities as follows: age <35 years without any comorbidity: annually; age <35 years with comorbidities: twice a year; and age >35 years: twice a year. For screening and monitoring metabolic disorders in PLHIV, blood chemistry testing is recommended within the first 3 months after starting or changing ART and every 3 months during the first year 14, 15, in contrast to the benefit pakage for HIV in Thailand. Moreover, adverse effects of ART were manifested differently in different races 16. Therefore, strong evidence from the local database is needed to challenge the revision of the laboratory screening, especially in the aging HIV population. Is the national health security for metabolic abnormalities among the Thai PLHIV appropriate? The study hypothesis is that there is a high occurrence of metabolic abnormalities in PLHIV and that there are other predisposing factors of metabolic abnormalities apart from age. This retrospective study aims to determine the occurrence of metabolic abnormalities and predict factors responsible for metabolic abnormalities among patients with HIV.
Methods
This retrospective study was conducted at the internal medicine clinic at the outpatient department of Pathum Thani Hospital, from September 2018 to Febuary 2019. The study was conducted in accordance with the principles for human experimentation, as defined in the Declaration of Helsinki and approved by the hospital ethics committee on 06 September 2018 (approval number: 0032.203.3/16076). The privacy of the research participants was ensured with an adequate level of confidentiality. The study conducted in the South West region of Cameroon showed that the highest prevalence of metabolic component in patients treated with the first-line HAART group and the ART-naive group were 53.2 and 14.8%, respectively 9. In order to determine the occurrence of metabolic abnormalities in patients with HIV after first-line HAART, the incidence was estimated to be 33% with a 95% CI and the precision to be within 5% of the true value. The sample size calculation was as follows:
where
n = sample size
z = 1.96 (α = 0.05)
p = the prevalence impaired fasting glucose
d = error allowance
Therefore, a sample size of at least 339 patients with HIV was required for this study.
Intensive training was provided for two data collectors, Pharm D students, using a set of practice medical records before the study started. The electronic predefined case record forms (eCRFs) were prepared for data collection with the electronic log book for patients’ confidentiality and patient tracing. The electronic medical records were screened by a medical statistician according to inclusion and exclusion criteria before random sampling by data collectors. Patients with HIV having had at least 1 year of the first-line ART, and having FPG, fasting lipid profile, and blood pressure assessment before starting ART were recruited into the study. Those with any abnormal metabolic component prior to ART or absent history of ART were excluded. Clinical characteristics were obtained from electronic medical records and laboratory data were obtained from the electronic laboratory report using the eCRFs. The scheduled meetings were arranged to resolve data clarification and conflicts.
The outcomes of interest
The outcomes of interest were the occurrence of metabolic abnormalities after at least 1 year of the first-line HAART and its predicting factors among patients with HIV.
Definition
The metabolic abnormalities were defined as at least one metabolic component of the following: blood pressure over 130/85 mmHg or hypertention, fasting triglyceride level over 150 mg/dL or dyslipidemia, and fasting blood sugar over 100 mg/dL or DM 17.
Statistical analysis
Data analysis was performed by Statistical Package for the Social Sciences 15.0 (SPSS, Chicago, IL). Categorical variables were summarized as frequencies and percentages, and then analyzed using the chi square test or the Fisher’s exact test. Continuous variables were summarized as mean ± standard deviation (SD) or median and interquartile range (IQR) values, and compared using t-test or the Mann–Whitney U-test where appropriate. Then, all variables with statistically significant relationships with metabolic abnormalities were included in the logistic regression model. The variables with statistical significance in this model indicated “the predictor” to the metabolic abnormalities. All tests for significance were two-sided, and P < 0.05 was considered to be of statistical significance.
Results
A total of 340 patients were recruited into this study. The first patient was recruited on 10 September 2018 and of the last patient was recruited on 11 January 2019. After at least 1 year of first-line ART, 30% (102/340) of patients had metabolic abnormalities. The demographic characteristics of patients with and without metabolic abnormalities were shown in Table 1. In both the groups, most of the patients were male. Patients with metabolic abnormalities were significantly older than those without metabolic abnormalities (42.45 ± 10.34 years old vs. 38.76 ± 10.24 years old; P value = 0.003). Proportions of those who were married (45.1% vs. 30.5%; P = 0.010) and had an occupation (100.0% vs. 94.5%, P value = 0.016) were significantly higher in patients with metabolic abnormalities. Traditional risk factors such as smoking (9.7% vs. 17.2%; P value = 0.139) and alcohol consumption (9.7% vs. 17.3%; P value = 0.134) were slightly higher in those without metabolic abnormalities while weight differences between the latest measurement and before ART initiation was slightly higher in the metabolic abnormality group (4.9% ± 10.36 vs. 3.9% ± 10.74; P value = 0.570). Furthermore, these patients had significantly longer average duration between diagnosis and initial treatment (4.21 ± 0.82 years vs. 4.05 ± 0.81 years; P value = 0.008) as well as ART duration (43.03 ± 10.05 months vs. 41.55 ± 9.27 months, P value = 0.018). Baseline blood chemistries and CD4 were similar in both groups.
Table 1. Bivariate Analysis of Demographic and Clinical Characteristics Associated with Metabolic Abnormalities after ART (n = 340).
| Variables | Total (n = 340) |
Metabolic abnormalities (n, %) | P | |
|---|---|---|---|---|
| No (n = 238) | Yes (n = 102) | |||
| Male | 200 (58.80) | 144 (60.90) | 55 (53.90) | 0.700 |
| Age (yeara) | 39.76 ± 10.75 | 38.76 ± 10.24 | 42.45 ± 10.34 | 0.003* |
| Married | 118 (34.90) | 72 (30.50) | 46 (45.10) | 0.010* |
| Having an occupation | 372 (96.20) | 225 (94.50) | 102 (100.00) | 0.016* |
| Smoking | 35 (14.90) | 28 (17.20) | 7 (9.70) | 0.139 |
| Alcohol | 35 (15.00) | 28 (17.30) | 7 (9.70) | 0.134 |
| % Weight changea,b | +3.96 ± 10.74 | +4.98 ± 10.36 | 0.570 | |
| Having comorbidities | 59 (24.70) | 39 (23.50) | 20 (27.40) | 0.519 |
| Having opportunistic infection | 135 (39.70) | 95 (39.90) | 40 (39.20) | 0.904 |
| Duration between diagnosis and initial treatment (yeara) | 4.01 ± 0.84 | 4.05 ± 0.81 | 4.21 ± 0.82 | 0.008* |
| ARTc duration (monthsa) | 41.14 ± 9.68 | 41.55 ± 9.27 | 43.03 ± 10.05 | 0.018* |
| Baseline CD4 (cell/mm3a) | 336.56 ± 242.14 | 327.88 ± 255.40 | 342.15 (219.80) | 0.429 |
aMean ± SD.
bWeight differences between the latest measurement and before starting ART.
cART, antiretroviral therapy
*statistical significance
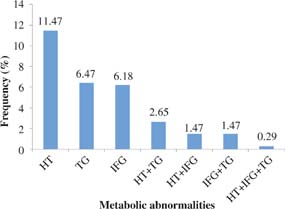
The distribution of metabolic abnormalities was shown in Figure 1. Of 102 patients, hypertension (39; 11.5%) was the single most common metabolic abnormality while the combination of hypertension and hypertriglyceridemia was the most common combination of metabolic abnormalities (9; 2.7%). MetS was found in one patient (0.3%).
Fig 1. The distribution of metabolic abnormalities (n = 102). HT, hypertension; IFG, impaired fasting glucose; TG, hypertriglyceridemia.
ART in these patients was similar except the proportion of patients treated with either efavirenz or nevirapine in the regimen containing lamivudine and stavudine, which was significantly greater in the metabolic abnormality group (3; 2.98 vs. 0, P value = 0.011) (Table 2).
Table 2. ART in HIV-infected Patients with and without Metabolic Abnormalities (n = 340).
| ART | Metabolic abnormalities after HAART (n, %) | P | |||
|---|---|---|---|---|---|
| NRTI | NNRTI | No (n = 238) | Yes (n = 102) | ||
| TDF | FTC | EFV | 111 (46.64) | 39 (38.24) | 0.554 |
| NVP | 1 (0.42) | 0 | |||
| 3TC | EFV | 57 (23.95) | 30 (29.41) | 0.442 | |
| NVP | 3 (1.26) | 3 (2.94) | |||
| AZT | EFV | 4 (1.68) | 1 (0.98) | 0.642 | |
| d4T | EFV | 1 (0.42) | 0 | ||
| ddI | EFV | 1 (0.42) | 1 (0.98) | ||
| 3TC | AZT | EFV | 29 (12.18) | 12 (11.76) | 0.762 |
| NVP | 25 (10.50) | 12 (11.76) | |||
| d4T | NVP | 6 (2.52) | 1 (0.98) | 0.011* | |
| EFV | 0 | 3 (2.98) | |||
ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine; AZT, zidovudine; d4T, stavudine; ddI, didanosine.
*statistical significanc
Logistic regression analysis showed that age was the single predictor of metabolic abnormalities in these patients (OR = 1.03, 95% CI = 1.00, 1.06, P value = 0.024).
Discussion
This is the first finding of the occurrence of metabolic abnormalities in patients with HIV after first-line ART in Thailand. About one-third of them had metabolic abnormalities, and it was likely to be underestimated due to the restricted benefit package for PLHIV. The fact that gender was of no consequence in patients with and without metabolic abnormalities supported a previous study in Thailand 18, 19. However, in Western and African countries, females are at a higher risk of MetS, compared to males 20, 21. Living with family and having an occupation tend to increase the occurrence of metabolic abnormalities in this study. Similarly, previous studies showed that marriage is associated with weight gain in both men and women and is strong predictor for MetS 22, 23. In addition, MetS was significantly increased in the unemployed 24. In line with a previous finding, no association was found between current smoking and alcohol consumption 25. The inconsistent research findings were found among non-HIV patients 26, 27. Mild-to-moderate alcohol consumption of beer and wine had a positive effect on lipids and fasting insulin 28. Moderate weight gain observed in our patients may increase the risk of MetS, as shown in a previous study 29. Generally, PLHIV are motivated to engage in health-promoting behaviors because intensive lifestyle modification significantly improved cardiovascular risk indicators in patients with HIV and MetS 30. Therefore, the traditional risk factors of MetS were found in small proportions and were not associated with metabolic abnormalities. Although there were significant differences in duration between HIV diagnosis and the initial treatment, and ART duration between the two groups, they were nonsignificantly different after logistic regression analysis. It was supported by a previous finding that the prevalence of MetS was not significantly different between longer and shorter durations of diagnosed HIV infection (32.0% vs. shorter: 19.1%, P = 0.251), as well as longer or shorter duration of ART (25.6% vs. 14.2%, P = 0.192) (10). CD4 cell > 350 cells/mm3 was a risk factor for dyslipidemia as well as CVD 31, 32, and low CD4 cell counts observed in this study showed no association with metabolic abnormalities in bivariate analysis. After 12 months of ART, abnormal lipid profile 33 and insulin resistance 34 were detected, the prevalence of which could be influenced by ART 35. NNRTI-based regimens were less likely to induce dyslipidemia 36. In addition, TDF, 3TC, and FTC had positive effects on lipid profile 37. However, hypertriglyceridemia was the second most metabolic abnormality found in our patients. Although ART containing didanosine and TDF were least commonly used in this study due to the risk of hyperglycemia 38, 39, hyperglycemia was found in 9.4% of the patients. In addition, increased risk of DM by NRTI and NNRTI was reported in a study of patients with HIV, mostly among black patients 12. Therefore, HIV infection may have a major impact on metabolic abnormalities in our patients.
Age was the single predictor of metabolic abnormalities, which was supported by a previous study 40, especially in patients treated with regimens not based on PI. In the era of HARRT, the life expectancy of PLHIV and people with age-related illness has increased 34. With increasing age, the risk of hypertension increased 41, which is supported by the highest rate of hypertension in this study. Furthermore, it is recommended to screen for hyperlipidemia and hyperglycemia at the inception of HIV care, after treatment initiation and modification 15, 17, 42, 43. However, laboratory testing provided by the benefit package for PLHIV is not sufficient for an early detection of all metabolic components. Delayed detection of metabolic abnormalities may lead to adverse cardiovascular outcomes. Our result highlighted that the national benefit package for aging patients with HIV should be revised in order to promote effective screening for metabolic abnormalities. FPG and/or HbA1c should be monitored within 1–3 months after initiation or modification of ART, and then once or twice a year 14, 44. In patients having impaired FPG or DM, FPG should be monitored every 3 months or HbA1c should be monitored every 6 months 14, 44. Fasting lipid profile should be monitored within 3 months after initiation or modification of ART, then every 3 months in the first year, and once or twice a year thereafter 15, 45. In patients having a triglyceride level of > 200 mg/dL, fasting lipid profile should be monitored within 3 months after initiation of ART 15, 45 and within 6–8 weeks after initiation or dose increases for statins, and subsequently every 6–12 months 46. Due to the economic burden in this resourse-constrained country, patients aged ≥ 35 years should be prioritized as the finding in HIV-1-infected Thai adults showed that they are at high risk for MetS 19. In addition, waist circumference and body mass index should be monitored at each visit. Lifestyle modification consisting of diet control, exercise, smoking cessation, and alcohol abstinence is strongly recommended 47.
There are some limitations to this study due to incomplete entries in the electronic medical records such as marital status, waist circumference, height, and weight. The metabolic abnormalities may be underestimated because of the limitation of laboratory testing according to national health security for PLHIV. Furthermore, the result might have limited generalizabiltiy as the study was conducted only among Thai patients with HIV.
Conclusion
Age-related risk factors should be monitored and treated in order to prevent or slow down metabolic disorders in PLHIV. Therefore, improvement of the benefit package for aging PLHIV, espectially blood tests for metabolic abnormalities, is urgently needed to promote a better quality of life and to diminish the long-term cost of health services.