INTRODUCTION
Mucormycosis is an angio-invasive fungal infection related to a high percentage of morbidity and mortality, mucorales is the fungi that cause mucormycosis.1 The incidence of mucormycosis increases in patients with diabetes mellitus, corticosteroid therapy, organ transplants, and haematological malignancy.2 In the Asian continent, diabetes mellitus is considered the most common risk factor.3
The most common form of this fungal infection is Rhino-orbital-cerebral mucormycosis (ROCM).4 It spread from the orbit to the brain and takes place via contiguous or hematogenous dissemination and/or to the cavernous sinus via venous drainage.5
There is a global increase in the incidence of mucormycosis, but the rise is very high among patients with uncontrolled diabetes mellitus in India and China.6
The mucorales spores germinate in patients with COVID-19 which is facilitated by the following primary reasons: hypoxia, diabetes, metabolic acidosis and decreased white blood cell phagocytic activity because of the immunosuppression and association with other risk factors such as prolonged hospitalisation.7 Mucormycosis in a diabetic patient may be fatal and causes severe complications if not treated timely and adequately.8
Mucorales is neutralized by the immune system through phagocytosis, chemotaxis, and killing by the oxidative and non-oxidative mechanisms. In diabetes mellitus, the patients lack these normal functions of immune cells and are considered in an immunocompromised state.9
Unfortunately, treatment with corticosteroids in patients of COVID-19 results in a dramatic outpouring of hyperglycemia and ketoacidosis, which permits the fungus to have a bountiful culture media.10
The nasal turbinates are considered the initial site for ROCMs, further aggressive proliferation occurs to involve the sinus, palate, orbit, and brain with a strong attraction for blood vessels.11 The spread of the infection from the sinus leads to osteomyelitic bone, further to the orbital content and brain by the orbital route. Spread of the infection to the cavernous sinus and sphenoid sinuses results in cranial nerves palsy.12 Facial pain, headache, swelling of nasal and periorbital regions, bleeding from the nose, loss of vision accompanied by facial paralysis, and nasal discharge that consists the amount of reddish-black nasal turbinate is considered the main manifestations of mucormycosis, if the patient left untreated, it will result in progression of the infection to the cranial region with many symptoms such asblindness, lethargy, and seizures usually followed by death.13
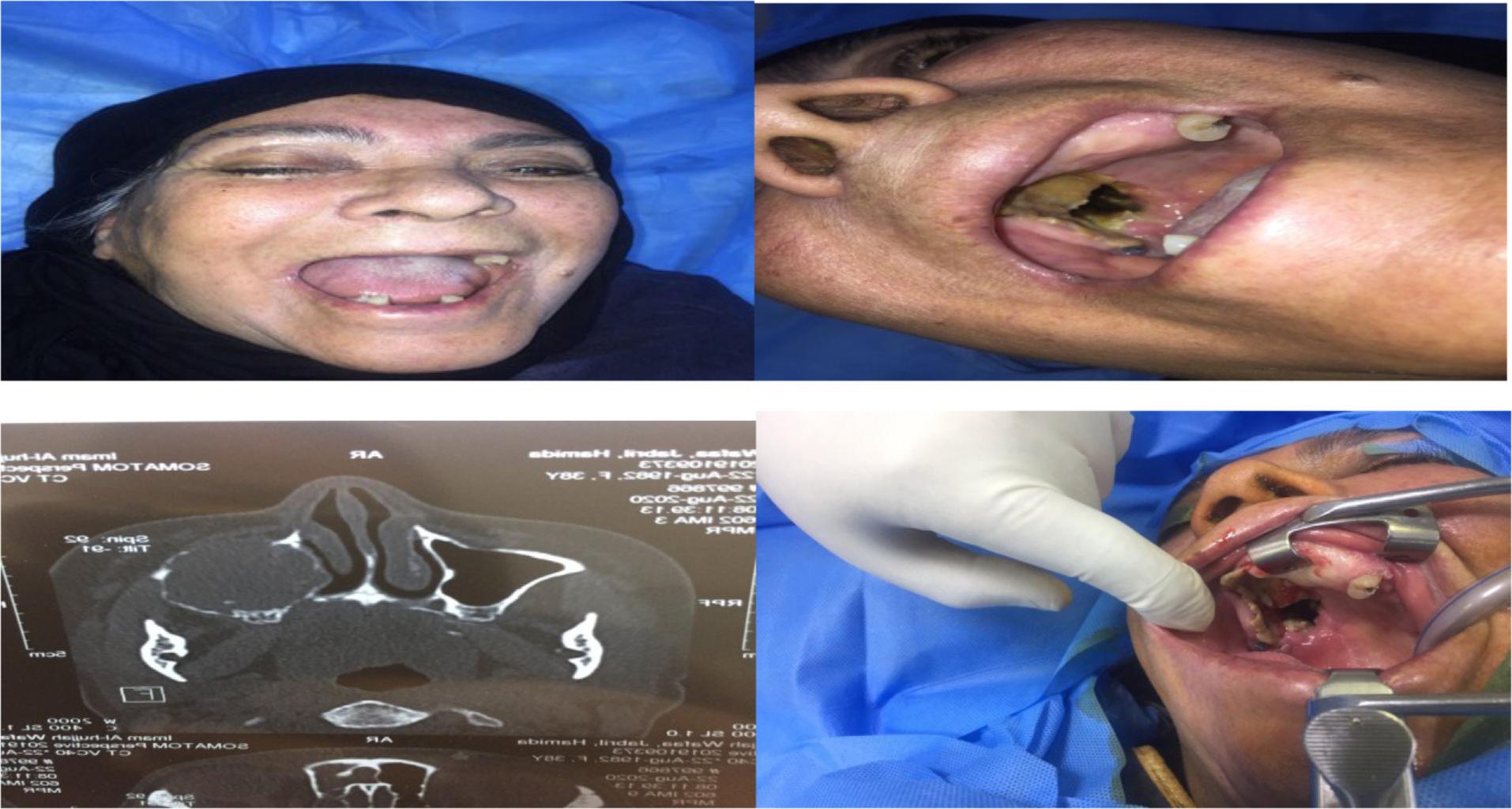
The diagnosis of mucormycosis is done by histopathological examination and/or fungal culture, sinus biopsies, tissues biopsies, and orbital tissue biopsies which are used as specimens for histopathology and culture examination.14 Extent of tissue involvement by mucormycosis is defined by the role of computed tomography(CT) scan and magnetic resonance imaging (MRI), which is very decisive for effective debridement of the involved tissue by infection (Figure 1).15 Difficulties in the early diagnosis, pathogenetic mechanisms, and differences in host–fungus interactions had been related to the higher degree of difficulty to recuperate this desolating infection.16
64-years-old female patient with destructive palate and orbital extension as shown by clinical presentations and computed tomography.
A multimodal approach is considered a base for the successful management of mucormycosis which include the reversal of underlying predisposing factors, early administration of an optimal dose of active antifungal therapy, and removal of infected tissues.17 The first choice active antifungal drug is Amphotericin B.18 Destruction of the fungal cell walls is obtained by Amphotericin B which usually prescribed in higher doses (5–10 mg/kg), reduction in the risk of nephrotoxicity is obtained by administrating the liposomal formulation.10
Surgery must be very aggressive; the removal of necrotic tissues is extended until the perfused tissue is encountered by the surgeon. Excision of the palate, and nasal cartilage, and exenteration of orbital contents may be required in advanced cases of ROCM.19
The prognosis of mucormycosis leans on multiple factors including the patient’s general health at the time of diagnosis, the early detection, and the site of infection.20
MATERIALS, PATIENTS, AND METHOD
This study was done at the Oral and Maxillofacial Department of Al-Hussien Teaching Hospital in Kerbala Governorate, Republic of Iraq. From the period extended from January 2019 until the end of December 2021 (this period of time was divided into two divisions: One before the declaration of COVID-19 as a pandemic in March 2020; from January 2019 until the declaration of the pandemic on 11 March 2020, another from time of declaration of the pandemic to the end of December 2021).
DIAGNOSIS
Clinical Examination
Clinical examination was done by MDT (multidisciplinary team) that involved a maxillofacial surgeon, ophthalmologist, otolaryngologist, neurologist, and internal medicine physician. Clinical examination was done carefully, especially in patient with clinical manifestations like palatal ulceration, palatal black discoloration, sequestrum formation in the maxillary alveolus and palate, patches of black discoloration over the skin of the nose, diplopia, periorbital erythema, and cellulitis. Pupillary reaction test and ocular motility were also included in the clinical examination.
Laboratory Tests
Once clinically suspected, routine laboratory tests were done that include: complete blood count, fasting blood sugar, HbA1c (glycated hemoglobin), and kidney function test including electrolytes.
Diagnostic Procedure
-
Diagnostic nasal endoscopy
-
Contrast enhanced CT for paranasal sinuses and nose
-
Contrast enhanced MRI for the brain, orbit, and face.
-
A biopsy that reveals angioinvasion, hemorrhagic infarction, and coagulation necrosis
-
Culture media (fungal culture)
Management
-
Strict control of blood sugar levels
-
Antibiotics (when there was superadded infection evidence only).
-
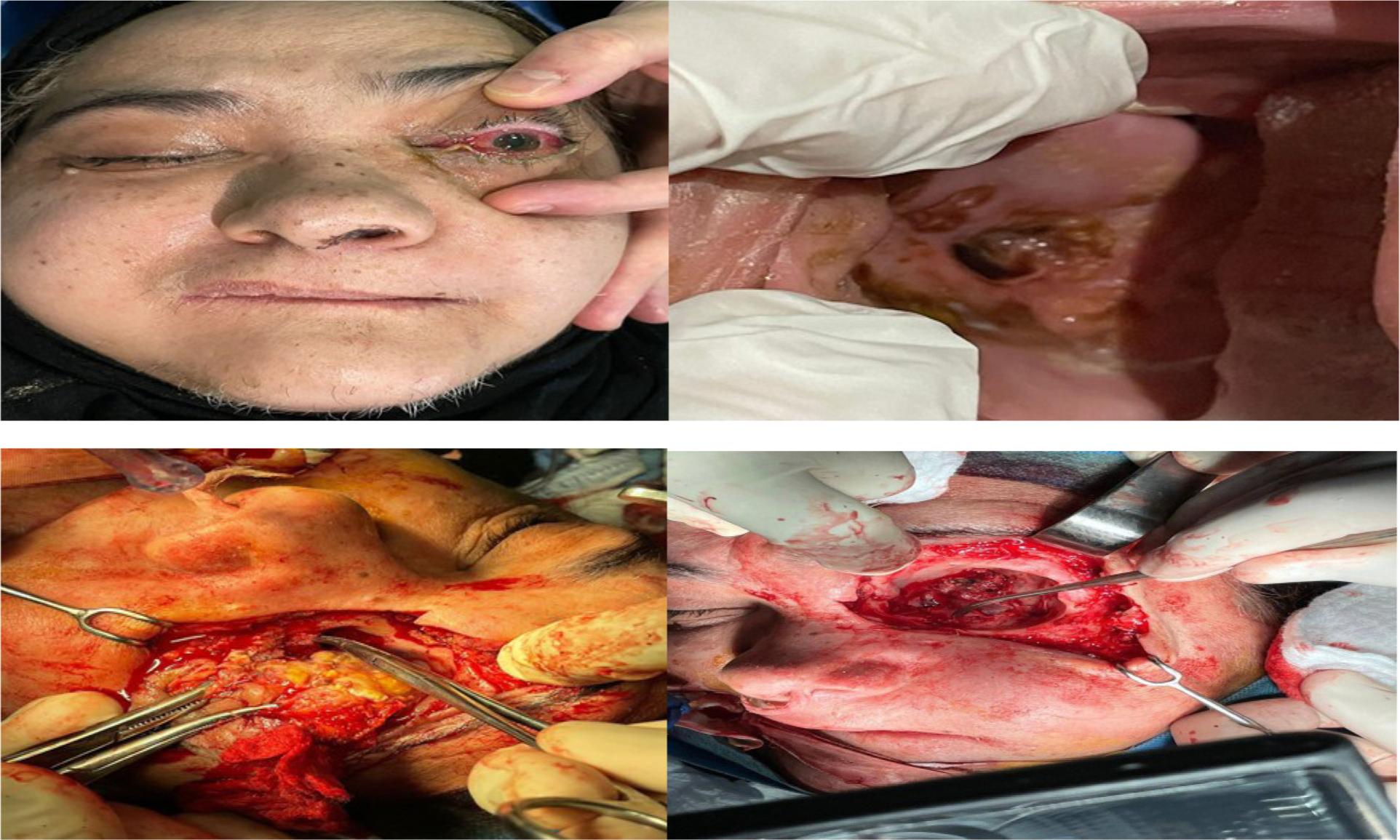
Extensive surgical debridement for the emoval of all necrotic tissues that involve fungal infection, which included (alveolar process with teeth resection, Cald well-Luc approach in maxillary sinus involvement, palatal resection by open maxillectomy, partial maxillectomy to total maxillectomy, resection facial skin and muscles, and orbital exenteration). Images from the CT and/or MRI helped in defining the extent of surgery. Extension of surgical detriment was determined by MDT (Figure 2).
59-year old female patient with mucormycosis with orbital (eye involvement) and palatal ulcerations, was treated by resection facial skin and muscles and orbital exenteration.
Antifungal Drugs
-
D-AmB (Amphotericin B deoxycholate) was described in the dose of 1–1.5 mg/kg/day that continued for3–6 weeks
-
L-AmB (Liposomal Amphotericin B) was described in the dose of 5–10 mg/kg/day that continued for3–6 weeks, L-AmB was used when it was available or when the use of D-AmB should be avoided according to the kidney function test.
METHODS FOR PREPARATION AND USING ANTIFUNGAL MEDICATION
Amphotericin B Deoxycholate (D-AmB)
-
Before each controlled infusion of Amphotericin B., one liter of normal saline with one amp. of KCL (20 mEq of potassium chloride) over 2 hours was given to the patients.
-
Each vial (50 mg of D-AmB) was reconstituted with 10 mL of injection water and should be immediately shake well to produce a colloidal solution with 5mg/mL.
-
Further dilution was obtained by 5% dextrose (500 mL to reach the concentration of 100 μg//mL.
-
Infusion was done over 2–4 hours or longer if not tolerated (10 mL was the initial test dose that means 1 mg over 20–30 minutes ).
-
After dilution, the infusion began immediately and was covered with a black sheet to protect it from light.
-
Flush existing intravenous line with glucose 5% was done because the drug is incompatible with sodium chloride solution.
Liposomal Amphotericin B(L-AmB)
-
Avial (50 mg of L-AmB) was reconstituted with 10 mL of injection water and to generate the liposomes the solution should be shaken well for2–5 minutes .
-
After the reconstitution of all the vials, 10 mL syringe was filled with reconstituted L-AmB.
-
The needle was removed and the syringe nozzle was applied with 5-micron filter and the content was emptied into 5% dextrose (200 cc).
-
Infusion of L-AmB over 2–3 hours was done (1 mg over 10 minutes was the initial dose)
-
Flush existing intravenous line with glucose 5% was done because the drug is incompatible with sodium chloride solution
RESULTS
Total 39 patients participated in this study; among them,21 were males and 18 were females. Patients were divided into three age groups, <30 years, 30–60 years, and >60 years, greater incidence was recorded in 30–60 years group (Table 1).
TABLE 1. Age and gender distribution for patients and comorbid conditions.
| Total no. 39 | Male | Female | ||
|---|---|---|---|---|
| 21 (48.85%) | 18 (41.15%) | |||
| Age of patients | Age groups | No. | Male | Female |
| <30 years | 2 | 2 | _ | |
| 30_60 years | 30 | 16 | 14 | |
| >60 years | 7 | 3 | 4 | |
| Comorbid condition | Type | No. | Male | Female |
| Diabetes mellitus | 35(89.76%) | 19 | 16 | |
| Glucocorticosteroids drug | 2(5.12%) | 1 | 1 | |
| Chemotherapy | 2(5.12%) | 1 | 1 | |
Mucormycosis and Association with COVID-19
Before the declaration of the pandemic, there were 7 patients, while after the spread of COVID-19, there were 32 patients which is about 4.5-time increase in the disease after the pandemic (Table 2).
TABLE 2. Types of mucormycosis and its relation to COVID-19.
| With history of COVID-19 | 32 | M : 17 | |
| F: 15 | |||
| Without history of COVID-19 | 7 | M : 4 | |
| F : 3 | |||
| Mucormycosis onset | Time of onset of COVID-19 | No. | Gender |
| At the time of diagnosis | 5 | M: 3 | |
| F: 2 | |||
| After admission in hospital | 12 | M: 7 | |
| F: 5 | |||
| After recovery | 15 | M: 7 | |
| F: 8 | |||
| Type | Type according to anatomical region involved | No. | Site |
| Oro antral mucormycosis | 15 | Palate, alveolar process, maxillary sinus | |
| Rhino sinus mucormycosis | 11 | Nose, maxillary sinus | |
| Rhino-orbital-cerebral mucormycosis (ROCM) | 13 | Nose, maxillary sinus, orbit, cranial cavity | |
Clinical Manifestations, Investigations and Management
All the patients who participated in this study were examined through their history, careful clinical examination, radiological examination (CT scan and/or MRI), culture and biopsy, and all of them were managed by aggressive surgical debridement and high dose of antifungal therapy (Table 3).
TABLE 3. Clinical manifestations of different type of mucormycosis, investigations, and management.
| Site of mucormycosis | No. | Comorbid condition | Clinical manifestations (all or some) | Investigations | Surgical debridement | Antifungal therapy |
|---|---|---|---|---|---|---|
| Oro-antral mucormycosis | 15 | 12 Diabetics, 2 on steroids and 1 on chemotherapy | Teeth mobility, gingival ulceration, palatal ulceration, dental pain, necrotic alveolar bone (sequestrum) facial pain, nasal blockage, para-sinusal pain | CT scan, biopsy and fungal culture – direct smear | Aggressive surgical debridement (alveolar bone resection with mobile teeth, curettage of maxillary sinus or partial maxillectomy) | Amphotericin B or liposomal Amphotericin B (according to availability) |
| Rhino-sinus mucormycosis | 11 | 10 Diabetics and 1 on chemotherapy | Black colored turbinates, erythema of nasal mucosa, stuffy nose, facial pain, nasal blockage, para-sinusal pain, facial erythema | Diagnostic nasal endoscopy, CECT for nose and paranasal sinus and biopsy | Aggressive surgical debridement of necrotic tissue, alveolar bone resection or partial maxillectomy | Amphotericin B or liposomal Amphotericin B (according to availability) |
| Rhino-orbital- cerebral mucormycosis (ROCM) | 13 | 13 Diabetics (all of them are uncontrolled | Diplopia, periorbital (erythema edema, cellulitis), corneal numbness, necrotic skin lesion (Escher), facial pain, black colored turbinates, black purulent discharge, change in level of consciousness, coma | Contrast enhanced magnetic resonance image (CEMRI) and biopsy | Aggressive surgical debridement of necrotic tissue (skin or muscle), partial maxillectomy, orbital exenteration. | Amphotericin B or liposomal Amphotericin B (according to availability) |
Patients Survival, Mortality and Prognosis
Death was recorded in four cases, survived patients were 35 after follow up for 6 months. In relation to prognosis, death was not recorded in period before declaration of pandemic COVID-19. All death cases were reported in period after declaration of pandemic and all of them were recorded in patients with ROCM (Tables 4 and 5).
TABLE 4. Survival, mortality and prognosis in patients with mucormycosis before pandemic COVID-19.
| Site of mucormycosis | No. of cases | No.of survival patients | No. of mortalities | Prognosis |
|---|---|---|---|---|
| Oro antral | 4 | 4 (100%) | 0 | Excellent |
| Rhino sinus | 2 | 2(100%) | 0 | Excellent |
| Rhino-orbital- cerebral (ROCM) | 1 | 1(100%) | 0 | Excellent |
| Total | 7 | 7(100%) | 0 | Excellent |
TABLE 5. Survival, mortality, and prognosis in patients with mucormycosis after the pandemic of COVID-19.
| Site of mucormycosis | No. of cases | No. of survival patients | No. of mortalities | Prognosis |
|---|---|---|---|---|
| Oro antral | 11 | 11 (100%) | 0 | Excellent |
| Rhino sinus | 9 | 9(100%) | 0 | Excellent |
| Rhino-orbital- cerebral (ROCM) | 12 | 8 (66.6%) | 4 | Moderate |
| Total | 32 | 28(87.5%) | 4 (12.5%) | Very good |
DISCUSSION
In our study, the increase in the incidences of mucormycosis and its association with COVID-19 had been shown by comparison between two periods before and after the declaration of COVID-19. Evidence-based review and literature search reveal that reports of mucormycosis from India and globally have observed a sudden upsurge recently with a definite relation with COVID-19.21 The study showed the occurrence of mucormycosis in the patient either in the active status of COVID-19 or after recovery. A large number of cases of mucormycosis were reported in patients with active COVID-19 infection as well as those post infections.22
The association between the incidence of mucormycosis and COVID-19, is explained by the effect of coronavirus on the immunological system of the infected patient which results in the impairment of cell mediated response,23 also the drugs that were used in the treatment of COVID-19, such as antibiotics and steroids. Patients that were diagnosed and treated for COVID-19 with corticosteroid therapies and broad-spectrum antibiotics are considered at the highest risk for developing fungal infection.24
The majority of patients who participated in this study had a history of diabetes mellitus, and they also had COVID-19, our study confirmed that diabetes mellitus was the main risk factor. In the international series reported by Hoengl et al., diabetes mellitus was the more predominant risk factor for coronavirus disease associated mucormycosis in the cases reported from India.25
Steroid therapy, which was used in the treatment of patient with COVID-19, is considered another risk factor for mucormycosis. Treatment of COVID-19 with steroid therapy can leads to an exacerbation of hyperglycemia and eventually leads to fungal infections such as mucormycosis, at the same time cause worsening of glycemic control in diabetic patients.26
The definite diagnosis of this fungal infection depended on the high index of suspicion especially in a patient with diabetes mellitus or on steroid therapy, clinical examination, quick evaluation of clinical symptoms, histopathology, direct microscopy, culture, CT-scan, and MRI. A quick evaluation of clinical symptoms, identification of host variables, and a strong index of suspicion are required for the diagnosis of mucormycosis.26 During the clinical examination of patients in this study, the intraoral or extraoral clinical manifestations like palatal ulceration, teeth mobility, sequestrum formation, unhealed extraction socket, nasal fistula, black nasal discharge, black discoloration, diplopia or periorbital pain, and cellulitis were considered indexes for high suspicion of mucormycosis especially in the patients with diabetes or history of COVID-19, and biopsies were done to confirm the diagnosis. Specimen from the surgical exploratory site is the only (invasive) option for the confirmation of diagnosis.27 All patients were sent for CT scan and/or MRI. When there was a clinical suspicion of mucormycosis, the diagnosis confirmation required the radiological examination, a CT of the orbit and maxilla should be done.28
After confirmation of diagnosis, our patients were treated immediately by antifungal therapy and aggressive surgical debridement, which depended on the extension of fungal invasion that was detected by radiological image (CT scan or MRI) and the decision of MDT, that varied in patients from alveolar process with teeth resection to the orbital exenteration. The extension of the surgical procedure is usually determined by the extent of the fungal infection, which is assessed by both clinical and radiographic examinations, CT scan and/or MRI.29 Aggressive surgical debridement of the area with fungal invasion was performed immediately when the diagnosis was confirmed. Survival rates were also improved by an aggressive surgical approach.30
An early aggressive surgical debridement of the infected craniofacial tissues is the foundation of successful treatment of rhino orbital cerebral mucormycosis; it includes resection of infected tissues of the face (skin and muscle), infected skin of the nose, maxillary sinus, and orbital exenteration.31
Management success and prognosis of mucormycosis in our study with 6 months’ follow-up, depended on early diagnosis, strict control of hyperglycemia, early administration of antifungal therapy, and aggressive surgical debridement. Immediate surgical debridement and early administration of antifungal therapy clearly improved the prognosis and decreased mortality.32 In the series reported by Hongil et al., mortality rates were lower in patients with ROCM who had adjunctive surgical treatment (4/28, 13.8%) versus patients treated by antifungals alone (5/8, 62.5%).25
Amphotericin B or Liposomal Amphotericin B were used for our patients, the selection of the drug depended on the drug availability and the state of renal function. A renal function test for monitoring blood urea and serum creatinine was done for patients on antifungal therapy. Rodriguez-Morales et al., recommend a high dose of liposomal amphotericin B as the first line of therapy whenever there is suspicion of mucormycosis.33 Amphotericin B deoxycholate was used in a dose of 1–1.5 mg/kg/day and Liposomal Amphotericin B was used in a high dose of 5–10 mg/kg/day, especially in ROCM. The recommended dose of liposomal Amphotericin B is 5 mg/kg/day.33
Death was recorded in our patients with advanced-stage fungal infection because of the incorrect diagnosis as the patient was initially was examined by a general dentist who had not enough experience or not familiar with the clinical features of this fatal disease. Systematic referral procedures for the specialist surgeon are essential for the improvement of prognosis and decrease the mortality. All death cases were reported in patients with ROCM with a history of uncontrolled glucose levels (diabetes), some of them were in coma because of a delay in the diagnosis that lead to the dissemination of fungal infection to the brain. Precious time was wasted by general dental practitioners who had a low index of suspicion for mucormycosis in performing mobile teeth extraction and root canal treatment leads to a delay in the inception of definitive mucormycosis treatment and thus resulted in a poor or fatal outcome for the patient.34
CONCLUSION
Mucormycosis is strongly associated with COVID-19, and the high number of cases had been reported after the declaration of the pandemic, especially in the second wave. Diabetic patients were at great susceptibility to developing mucormycosis and diabetes mellitus was the main risk factor in our study. A better prognosis was obtained by early diagnosis, accurate diagnostic procedures, early initiation of antifungal therapy, and aggressive surgical debridement.