INTRODUCTION
The superfamily of protein tyrosine phosphatases’ (PTPs) prototype PTP 1B has been connected to several signaling pathways.1 PTPs may provide a new class of therapeutic targets since certain particular PTPs, including PTPN1, PTPN2, PTPN9, PTPN11, PTPRF, PTPRS, and DUSP9, are linked to the inhibition of insulin signaling and cause insulin resistance that is important for the onset of type-2 diabetes.2 The conserved intracellular energy sensor known as AMP-activated protein kinase (AMPK) controls the metabolism of glucose and lipids. When activated, AMPK promotes glucose uptake and lipid oxidation in skeletal muscle and adipose tissues.3
The widely used hypoglycemic medications metformin and thiazolidinedione increase AMPK activity, showing that AMPK is a marker for an anti-diabetic effect.4 PTP, non-receptor type 1 (PTPN1, also known as PTP1B), which is connected to the negative regulation of insulin action, raises the possibility that inhibiting PTPN1 might be a therapeutic approach for the management of type 2 diabetes.5 PTP1B, a non-receptor protein phosphatase that phosphorylates tyrosine, is regarded as a key negative regulator of both insulin- and leptin--simulated signal transduction. Previous research has shown that the absence of PTP1B can increase insulin sensitivity, improve glycemic management, and protect against the obesity-inducing effects of high-fat diets.6
Additionally, treating diabetic mice with PTP1B antisense oligonucleotides might lower PTP1B expression levels, regulate blood sugar levels, and ultimately improve insulin sensitivity.7 PTP1B inhibitors may improve insulin and leptin sensitivity and function as efficient treatments for type II diabetes, insulin resistance, and obesity, according to some research.8 Gene-targeting experiments in mice have identified PTP1B as a crucial physiological regulator of metabolism by attenuating insulin, leptin, and growth hormone signaling, making it a promising therapeutic target for type II diabetes and obesity.9
It appears that PTP1B function is not necessary for embryonic development. The two main -metabolic disorders in industrialized societies – -diabetes and obesity – are resistant in PTP1B-deficient animals, nevertheless.10 It should come as no surprise that the pharmaceutical industry holds PTP1B in high esteem as a target for the therapy of these illnesses.11
Both PTP, non-receptor type 9 (PTPN9, also known as PTP-MEG2) and PTP, non-receptor type 11 (PTPN11, also known as SHP-2), were identified as promising anti-diabetic targets, and chebulinic acid was shown to be their targeted inhibitor.12 The use of inhibitors against PTPN1, PTPN9 or PTPN11 is regarded as an effective method for treating type 2 diabetes since it has been demonstrated that they enhance insulin sensitivity and the PTP association with insulin resistance.13
Free fatty acids (FFAs) that play a significant role in insulin control and beta-cell activity include palmitic acid (PA), which has been demonstrated to stimulate mTOR signaling in rat hepatocytes.14 Increased saturated FFA concentrations influence insulin biosynthesis secretion, cell content, and also cause cell stress. This, in turn, causes lipotoxicity, which may cause cell function to be lost, and plays a direct role in the pathophysiology of T2D.15 FFAs affect the control of insulin via binding to their primary receptor, FFA receptor 1 (FFAR1), also known as GPR40.16 G-protein coupled receptor FFAR1, which primarily expresses in pancreatic beta-cells, has seven transmembrane domains.17 Different medium- and long-chain (C12–C22) FFAs activate cells, causing a rise in intracellular calcium levels and the stimulation of insulin secretion, which increases the insulinotropic capability of glucose and amplifies glucose-stimulated insulin secretion (GSIS). However, it is still unknown how exactly FFAR1 works.18,19
Insulin secretion, insulin resistance, and glucose absorption have all been identified as the mechanisms of action underpinning the reported anti-diabetic benefits. Although there are several distinct groups of polyphenols for the treatment of diabetes that have not been completely researched, the quest for novel therapeutic targets continues to be difficult. Overall, this research sought to determine the total fatty acid content of Ballota saxatilis extracts and to assert potential antidiabetic properties using an in silico approach by simulating the interactions of linolenic and PAs with target cell signaling proteins involved in the onset of diabetes.
MATERIAL AND METHODS
B. saxatilis leave was purchased from the neighborhood market in Baghdad Province, Iraq, for the extraction of biologically active chemicals. The plant was thoroughly cleansed of any other herbs, washed, and then gently dried on paper towels. In the meantime, dried plant blooms were harvested using paper bags and stored for roughly 30 days in a dark environment at a temperature of 25°C.17
Finely powdered dried leaves were sieved through a 0.4-mm mesh panel after being pounded into powder. According to the aforementioned procedure, alfalfa aqueous extracts were made using water, ethanol, and other ingredients. A sample of 500 g of dried alfalfa flower powder was extracted with 100 mL of 99% ethanol or water and left on a water path that had been heated to 60°C for - 20 minutes. The extract was recovered using a vacuum filtering assembly, and the material from the extract was dried using a rotary evaporator. The finished powder was weighed and kept at 4°C in a tight container until use.
Mass Spectrometry Using Gas Chromatography
Using the gas chromatography technique (GC) (Chrompack-Packard 438A) and a separation column type 30-SE with an inner diameter of 0.25 mm and a length of 30 m, according to the method described in, the biologically active chemicals were found in B. saxatilis extracts.17
Research on Molecular Docking
To determine which ligand molecule and the researched PTPs fit together the best, the key hypothesis and locking were employed. The crystal structure of the protein was reconstructed by RCSB PDB(protein data bank) which provides a variety of tools and resources for studying the structures of biological macromolecules. File then imported into molegro virtual Docker (VMD) the visual Molecular Dynamics that utilize a molecular modelling and visualization computer program. PA and α-Linoleic acid (ALA) were used as ligands and described from B. saxatilis. The ligand receptor’s binding mechanism did not include water molecules. Water molecules were deleted to prevent their additional H-bonding in order to optimize computation and avoid potential distortion following a docking procedure.18
Preparation of Biologically Active Chemicals
In the current investigation, various bioactive substances were employed. These substances were examined in aqueous extracts of dried leave from the plant B. saxatilis. Then, the ligands were produced for docking along with the MVD molecules. The 3D ligand structures were created with assistance from UCSF, while the 2D structures were taken from the ZINC15 chemical database. In order to build ligands, the UCSF Chimera Structure Build module was employed (minimizes energy consumption, adds hydrogen atoms, and adds charges when needed). The 3D structure of each drug was created, saved in pdb format, and then optimized for docking utilizing UCSF Chimera tools.
RESULTS
The development of rational structure-based medicines frequently makes use of contemporary techniques like molecular docking. It is used to predict the intensity of the forces at play, evaluate the complex interactions between small ligands and massive macromolecules, and pinpoint the optimal geometric arrangements.
Molecular docking revealed that PA had strong binding affinities for PTP1B (−7.8) and PTP9 (−7.9) but had weaker affinities for PTP11 [up to (−7.4. ALA)]. It produced close results of binding activity against PTP1B (−6.2) and PTP9 (−6.1) and lower binding activity reacted with PTP11 (−5.7) (Table 1).
TABLE 1. The interaction between the protein tyrosine phosphatase 1 band ligand α-Linoleic acid and palmitic acid.
| Complex | Binding affinity | Interacting residues | H-Bond interaction | Distance |
|---|---|---|---|---|
| PTP1B-ChEBI_15756 (Palmitic acid) | −7.8 | – | – | – |
| PTP1B_ChEBI_27432 (α-Linoleic acid) | −6.2 | Gly220(A) Phe182(A) |
O1-N O2-N |
3.22 3.17 |
From stimulation data we recognized that PA and ALA could interact through different type of linkages with the protein molecule of enzyme PTP1B-ChEBI as presented in Figure 1A and B.
FIG 1. The contact between protein PTP1B and the ligand (A) palmitic acid (B) α-Linoleic acid.
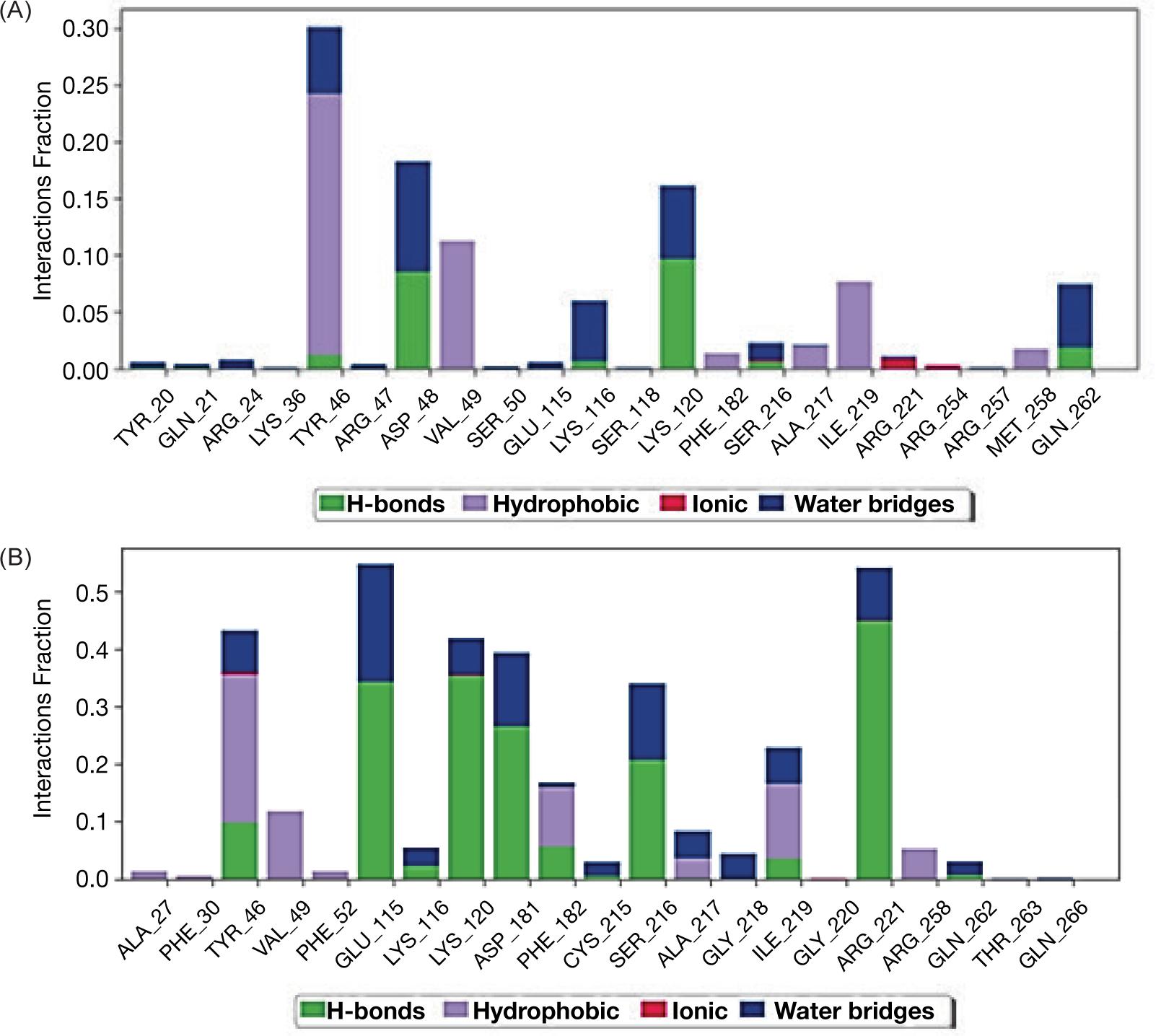
The molecular dynamics of protein with ligands showed that ligand PA with higher affinity inserted itself through hydrophobic interaction but without intra-hydrogen bonding with protein molecule while, ligand ALA interacted via hydrogen bonds with PTP1B protein at glycine 220 with distance 3.22 A and the amino acid phenyl alanine 182 with distance 3.17A as shown in Figure 2A and B.
FIG 2. Schematic figure represents molecular dynamic of interaction between the enzyme PTP1B and ligands palmitic acid (A) and α-Linoleic acid (B).

Our results indicated the interaction between protein molecule and ligand PA (A) and the second ligand ALA (B); both ligands interacted with PTPN9 via different bonds (Table 2 and Figure 3A, B).
TABLE 2. The interaction between the protein tyrosine phosphatase non-receptor 9 and ligand α-Linoleic acid and palmitic acid.
| Complex | Binding affinity | Interacting residues | H-Bond interaction | Distance |
|---|---|---|---|---|
| PTPN9_ChEBI_palmitic acid | −7.9 | – | – | – |
| PTPN9_ChEBI- α_Linoleic acid | −6.1 | Glu313(B) Arg551(B) |
O2-O O1-NE |
3.17 3.07 |
FIG 3. The contact between protein PTP9B and the ligand (A) palmitic acid and (B) α-Linoleic acid.
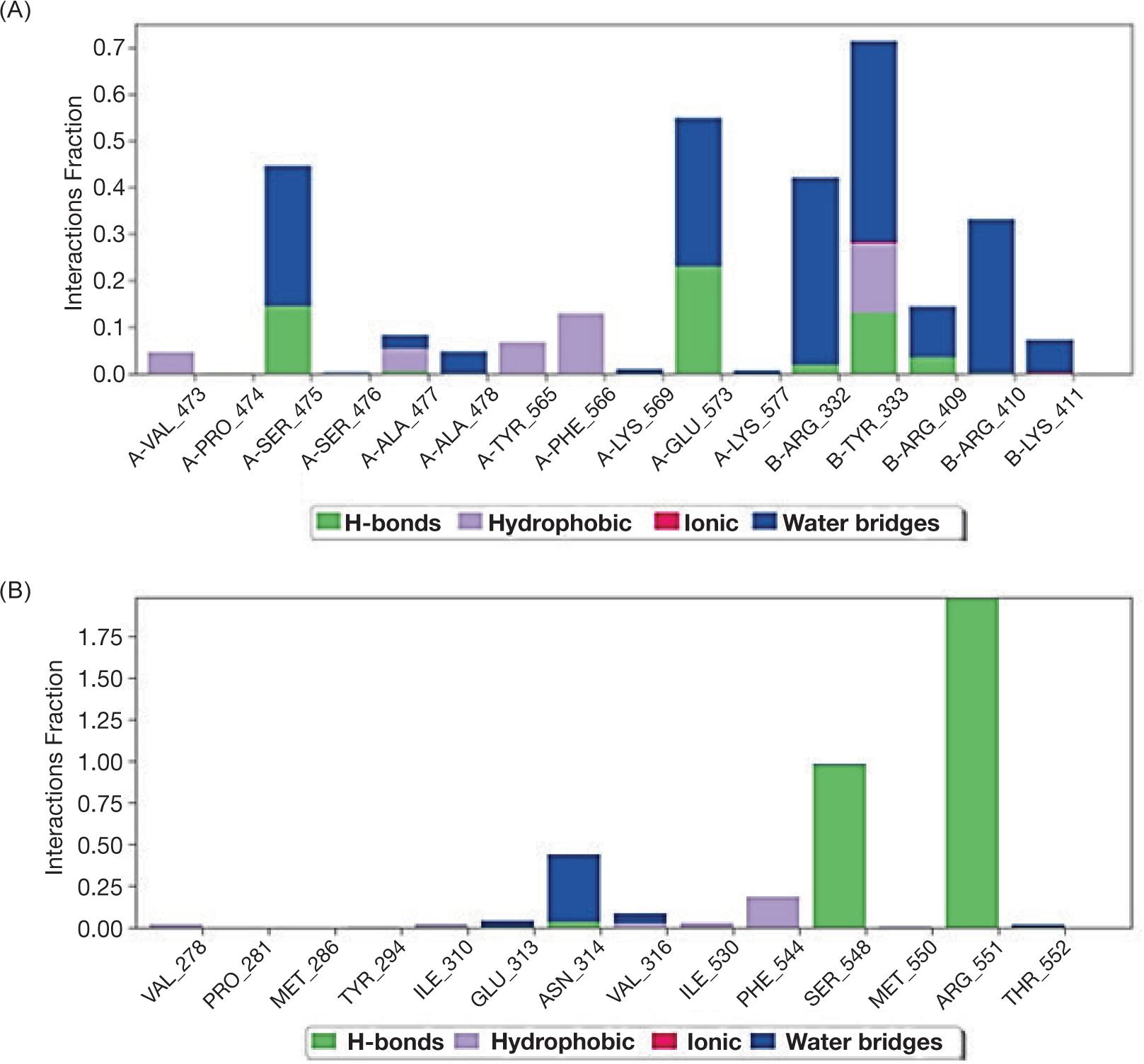
Despite the interaction, the molecular dynamic of protein PTPN9 with ligands indicated that ligand PA inserted by hydrophobic interaction without intra-hydrogen bonding with protein -molecule while, ligand ALA interacted via -hydrogen bonds at the position of amino acid Glu313 with distance 3.17A and the amino acid Arg551 with distance 3.07 Å as shown in Figure 4A and B.
FIG 4. Schematic figure represent molecular dynamics of interaction between PTP9 enzyme and ligand palmitic acid (A) and α-Linoleic acid (B).

The third target presented here was PTP11 that interacted with both ligands through the formation of hydrogen bonds at position Lys260 (A) and Asp477 (A) Table 3 and via different bonds (Table 3 and Figure 5A, B).
TABLE 3. The interaction between the protein tyrosine phosphatase non-receptor 11 and ligand Linoleic acid and palmitic acid.
| Complex | Binding affinity | Interacting residues | H-Bond interaction | Distance |
|---|---|---|---|---|
| PTPN11_ChEBI_15756 (palmitic) | −7.4 | Lys260(A) | O1-NZ | 2.99 |
| PTPN11_ChEBI_27432 (α-Linoleic) | −5.7 | Asp477(A) | O2-OD2 | 2.96 |
FIG 5. The contact between protein PTP11 and the ligand (A) palmitic acid and (B) α-Linoleic acid.

Molecular dynamics focused on the PTP11 protein ensured that hydrogen bond would occur between the amino acid Lysine 260(A) and ligand PA at distance 2.99A while hydrogen bond detected at position Asparagine 477(A) with distance 2.96A (Figure 6A and B); such finding was very interesting for targeting the protein molecule of PTP11 as a third option for diabetes treatment.
FIG 6. Schematic figure represents molecular dynamics of interaction between PTP11 enzyme and ligand palmitic acid (A) and α-Linoleic acid (B).

DISCUSSION
In addition to fat deposition and lipid buildup in the belly and visceral compartments, which cause age-enhanced insulin resistance or hyperinsulinemia, insulin resistance has been identified as a distinguishing characteristic of the natural aging process.20 Activation of the polyol pathway, increased production of intracellular AGOs like glycated red blood cells, activation of protein kinase C isoforms, and excessive activity of the hexose amine pathway are all effects of hyperglycemia. When these routes are combined, they enhance the generation of free radicals such as superoxide, hydrogen peroxide, and hydroxyl radicals, which have a negative impact on cells by increasing oxidative stress and harming microvascular endothelial cells. All of them contributed to the development of DM sequelae as diabetes retinopathy, nephropathy, and neuropathy and also the macrovascular issues such as stroke complications in type 2 DM, peripheral arterial disease, and coronary artery disease.20,21
Dietary habits were known to help type 2 diabetics by regulating their glycemic parameters; the American and Canadian Diabetes Associations advised eating more foods with unsaturated fats rather than saturated and trans fats. Numerous studies had recommended numerous species of the genus Ballota as a secure and affordable dietary supplement for a variety of illnesses.22 Founding by Ghaedi et al.23 concluded that Ballota glandulosissima can be used as a supplement by diabetics due to its inhibitory action against the enzyme α-glucosidase. While according to earlier research by Abdullah et al.24 on diabetic rats, the aqueous extract of B. saxatilis may have potential anti--hyperglycemic actions. This may be more potent than the prior anti-diabetic properties of other Ballota genus members like B. nigra and B. undulate.25 B. saxatilis was examined for its chemical composition using GC-Mass, and it was discovered that the extract contained palimitic acid and ALA, both of which were designed for in silico studies of their effects as antidiabetics against PTPs, which are an emerging paradigm for the development of anti--diabetic drugs.
Jiang et al.26 considered that PTP1B is a promising drug target for the treatment of diabetes type 2 as well as obesity through its action as a negative regulator of insulin receptor signaling and could had potent inhibitory activity against PTP1B.
Many researchers27–30 deal with the importance of the expression of the gene responsible for PTP1B protein expression and its relation with insulin resistance which leads to type 2 Diabetes. Other researches describe the mimetic of insulin through the function of non-selective inhibitors against PTP1B enzyme. However, other dephosphorates enzymes could activate insulin receptors. For this hypothesis, we conclude the PTP 9 as a second target for our extracts.
With its potential against insulin resistance, the enzyme PTP11 has previously been identified as a target for the treatment of diabetes. This protein is expressed in a variety of tissues and is essential for controlling many cell functions. PTP 11 regulates transcription, mitogenic activation, and cell migration in addition to metabolic regulation. Several phytochemicals have the ability to control either one or both of the molecules; omega-3 also increases fatty acid oxidation and decreases de novo lipogenesis, which results in less hepatic fat buildup and maintains hepatic insulin sensitivity. The release of glucagon-like peptide-1, which is regulated by GPR120, is another explanation put out to explain how omega-3 fatty acids have anti-diabetic effects.31,32 Numerous studies have shown that long-chain monounsaturated fatty acids and diets rich in linoleic acid encourage and enhance the release of GLP-1 in murine rat and human L cells, which raise levels of insulin in the blood.33
CONCLUSION
The first line of treatment for type 2 DM is oral anti-diabetic medication. In addition to insulin resistance, type 2 diabetes is also characterized by an increase in oxidative stress, which leads to the depletion of the cellular antioxidant defense system. Dietary habits are known to help diabetics with type 2 by regulating their glycemic parameters; the American and Canadian Diabetes Associations advised eating more foods with unsaturated fats rather than saturated and trans fats. Numerous in vitro and animal investigations have shown that ALA regulates insulin sensitivity by influencing glucose homeostasis through putative roles in gene regulation, fat metabolism, and adipocyte development. Various species of the genus Ballota have been recommended by numerous studies as a secure and affordable dietary supplement for a variety of ailments.
As a result, the present study recommends B. saxatilis as a brand-new oral hypoglycemic agent. The plant’s growing in the Iraqi environment and the use of its extracts in dietary regimens both require more research. From a substantial class of phytocompounds that are widely distributed and have demonstrated therapeutic activity against a number of clinical disorders, including neurological illnesses, PTPs, or protein tyrosine phosphatases, are a promising target for the creation of anti--diabetic medications.